kr lewis structure|6.1 Lewis Electron Dot Symbols : Cebu The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom and the number of . Watch 卑猥なボディのロリ巨乳保育士と連続大量中出し on SpankBang now! - Rori, Asian, Teens Porn - SpankBang
kr lewis structure,Lewis structure: Formalism used to show the structure of a molecule or compound, in which shared electrons pairs between atoms are indicated by dashes. Non-bonding, .

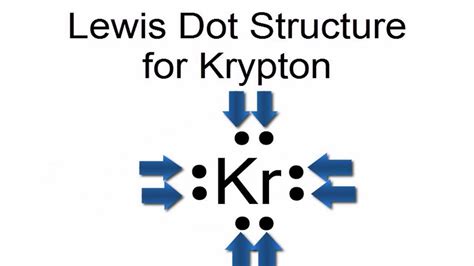
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.Lewis Structures. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules .
The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom and the number of .6.1 Lewis Electron Dot Symbols About. Transcript. A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around . Lewis structure of KrCl4 contains four single bonds between the Krypton (Kr) atom and each Chlorine (Cl) atom. The Krypton atom (Kr) is at the center and it is surrounded by 4 Chlorine atoms .kr lewis structureA Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of . The Lewis structure shows Kr in the center with two single bonds to two fluorine (F) atoms, each contributing 7 valence electrons. Kr uses two of its valence electrons for bonding, and the remaining six form three lone pairs. The molecule adopts a linear geometry with bond angles of 180°. This structure is a result of Kr’s ability to form .

Now in the KrF2 molecule, you have to put the electron pairs between the krypton atom (Kr) and fluorine atoms (F). This indicates that the krypton (Kr) and fluorine (F) are chemically bonded with each .
The Lewis structure of KrF2 shows that K is surrounded by 3 lone pairs of electrons and forms single bonds with each of the F atoms. . Kr belongs to group 8. Group 8 consists of noble gases which are . Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the . The lone pair over Kr is, 8-4 = 4. The lone pairs over Cl is, 7-1 = 6. So, the total lone pairs of KrCl 4 is 4 + (6*4) = 28 lone pairs of electrons. 6. KrCl 4 lewis structure angle. A bond angle is that angle makes by the atoms which are present in a molecule for proper orientation and shape.
A step-by-step explanation of how to draw the KrBr4 Lewis Dot Structure.For the KrBr4 structure use the periodic table to find the total number of valence el. Now in the KrF4 molecule, you have to put the electron pairs between the krypton atom (Kr) and fluorine atoms (F). This indicates that the krypton (Kr) and fluorine (F) are chemically bonded with each other in a KrF4 molecule. Step 4: Make the outer atoms stable. Place the remaining valence electrons pair on the central atom. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .
kr lewis structure|6.1 Lewis Electron Dot Symbols
PH0 · Lewis Structures
PH1 · Lewis Structure of KrCl4 (With 5 Simple Steps to
PH2 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols
PH3 · Lewis Dot Structures
PH4 · Lewis Dot Structure for Krypton Atom (Kr)
PH5 · How do you draw a Lewis dot structure of Kr?
PH6 · Drawing Lewis diagrams (video)
PH7 · 9.3: Drawing Lewis Structures
PH8 · 7.3 Lewis Symbols and Structures
PH9 · 6.1 Lewis Electron Dot Symbols